Newsweek
The more we learn and the more deeply we understand, the better we can improve our brain performance and increase our capacity to learn.

14 July, 2022
Before any product can be sold within the European market, it needs a market authorization (MA) from European Medicines Agency (EMA). Once a medicinal product gets […]
8 July, 2022
Today, TPI TV has turned 7 years old, the process is not too short nor long but enough for TPI TV to gradually mature and become more stable. Over the past 7 years, the number of members in the TPI TV family is increasing day by day, people come and go, but they all contribute to making TPI TV stronger and constantly innovating as it is now.
7 July, 2022
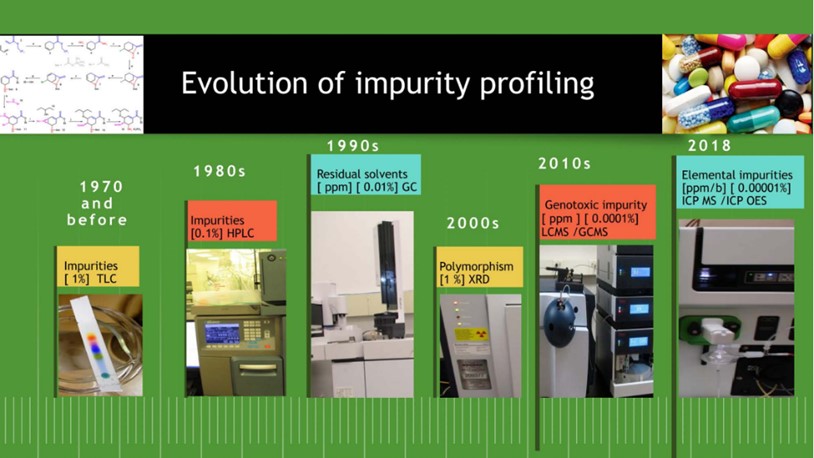
1. Introduction Impurity is considered as any component of a drug substance that is not a chemical entity defined as the drug substance and in addition, […]
27 June, 2022
Planning is one of the necessary skills in both work and life if you want to handle work effectively, and systematically, increase initiative, and control as […]
25 June, 2022
In June, in addition to “International Children’s Day” (June 01) which is an occasion to show affection to the preschool children of each family, we have […]
15 June, 2022
The following below contents do not contain the full and detailed information of the circular, but the information is extracted and summarized briefly as a part […]
14 July, 2022
Before any product can be sold within the European market, it needs a market authorization (MA) from European Medicines Agency (EMA). Once a medicinal product gets […]
8 July, 2022
Today, TPI TV has turned 7 years old, the process is not too short nor long but enough for TPI TV to gradually mature and become more stable. Over the past 7 years, the number of members in the TPI TV family is increasing day by day, people come and go, but they all contribute to making TPI TV stronger and constantly innovating as it is now.
7 July, 2022
1. Introduction Impurity is considered as any component of a drug substance that is not a chemical entity defined as the drug substance and in addition, […]
27 June, 2022
Planning is one of the necessary skills in both work and life if you want to handle work effectively, and systematically, increase initiative, and control as […]
25 June, 2022
In June, in addition to “International Children’s Day” (June 01) which is an occasion to show affection to the preschool children of each family, we have […]
15 June, 2022
The following below contents do not contain the full and detailed information of the circular, but the information is extracted and summarized briefly as a part […]