
Mean Kinetic Temperature – MKT
9 March, 2023
Multi-Unit Pellet Systems (MUPS)
29 March, 20231. Common definition(s)
Primary Standard (primary standard): A standard of the highest purity, having the important properties fully validated and widely recognized, of suitable quality under specified conditions, and having a recognized value acceptable without having to compare it with another standard.
Reference standards: including pharmacopoeial standards and non-pharmacopoeia standards (basic standards provided by the manufacturer)
• Pharmacopoeia standard: authentic substances of very high purity with critical characteristics and suitable for specific purposes are provided by official pharmacopeial agencies (Primary Standard).
• Non-pharmacopoeia standards: standards of very high purity, in-house compliance in the case of new substances or no pharmacopoeial standard reference available or not recommended. in the pharmacopoeia. They are also verified through independent testing methods with certificates from the manufacturer (Suppelco, Fluka, Sigma Aldrich, Merck).
Calibration Standard: authentic substances of high purity and suitable for calibration purposes and are provided by authorized sources.
Secondary standards (working standards): are high purity materials that are quantified within an acceptable range of the primary standard and are used on a daily testing by the quality control department (QC) in Pharmaceutical factory. Working standards are assigned a limited validity depending on the stability and shelf life of the material, new working standards must be prepared for future use. It is important to note that if an expired working standard is used in the analysis, the reliability of the reported results cannot be achieved.

2. Purpose
Primary standards normally come with a certificate of analysis and bear traceability to a globally recognized standards body. The cost is often too high for even milligram-range quantities and cannot use as the daily standard with a huge-experiments in Quality Control Department in Pharmaceutical Manufacturers. Therefore, traceability in the laboratory is of great interest to many people.
3. Process of tracebility standard in QC department
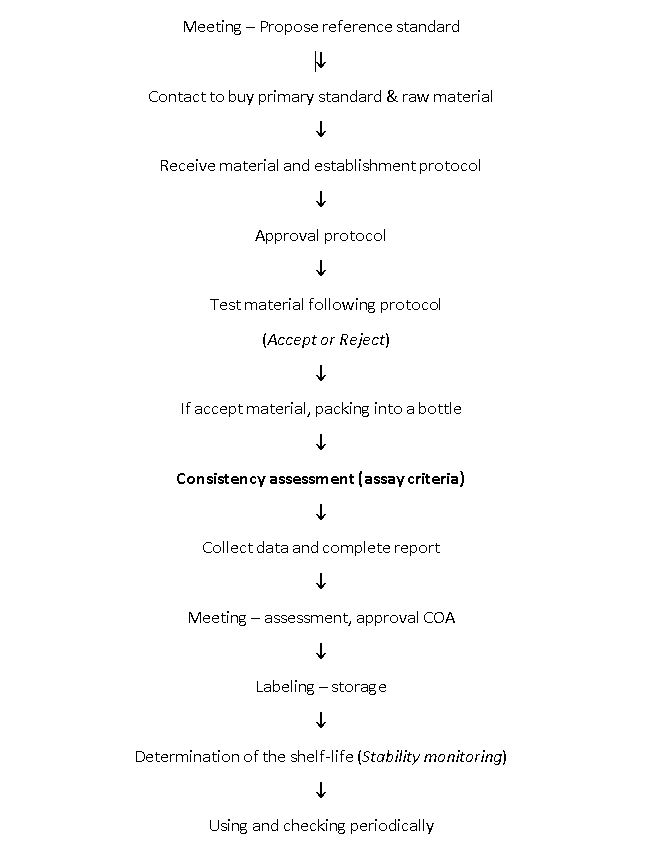
3.1 Packaging WS Regulation
- Prepare dry, clean brown glass vials, parafilm for wrapping the mouth of vial, and the raw material that needs packing.
- Packaging steps should be performed in Glove box for standard vial packing. The environmental condition for packaging is defined (temperature 22 ± 50C, humidity 45 ± 5%).
- Packing amount/ 1 vial depends on the standard amount weighing for assay or identification, it can be from a few tens to a few hundred milligrams but at least it must be enough for 2 times of weighing for test. Tighten the stopper, tightly wrap the mouth and cap with parafilm.
- The number of vials required packing/ 1 time depends on the actually used amount but must be enough until the expiry of the working standard, which normally is 12 vials.
3.2 Selection of Batch for working standards
- Select the latest approved batch of raw material for working standard having Assay value of more than 99.0% (Calculated on an anhydrous basis or on it states).
- Select the batch which does NOT have any deviation report or is out of specification (out of specifications – OOS) during sampling and testing.
- Collect a quantity of material for the preparation of working standards as per consumption of material.
- The new working Standards shall be prepared before the expiry of the existing working standard and given requisition to the warehouse department for raw material required.

3.3 Consistency assessment
Tests for the working standards
- Assay – 6 samples by 2 analyst
- Loss on drying/ water – 2/4 samples by 2 analyst
- Identification – 1 sample by 1 analyst
- Chromatographic Purity/ Related substances/ Related impurities
Consistency assessment of Working standard
- Sampling: Take 12 vials (12 test samples) randomly to perform assay testing items.
- Conditions of sample preparation and measurement: 3 ways
- 1 analyst prepares 12 test samples divided into 2 days, 6 test samples are prepared 1 day. Measure 2 days on 2 different equipment.
- 2 analysts prepare 12 test samples within 1 day, every analyst prepares 6 samples. Measure 6 samples/ equipment with 2 different equipment.
- 2 analysts prepare 12 test samples divided into 2 days, Every analyst prepares 6 samples. Measure on 1 equipment
- Conditions for sample preparation and sample measurement will depend on the quantitative method established in the previous protocol, including: chemical method, polar rotation method, potentiometric method or chromatographic method. high performance liquid chromatography, UV-Vis spectroscopy or microbiological methods.
- Requirement: relative standard deviation of assay results must meet requirement RSD% ≤ 0.5% (apply for assay with physicochemical method) or RSD% ≤ 2.0% (apply for assay with microbiology method).
- Measured and calculated results must be evaluated: Remove the outlier of assay results by the Dixon evaluation test with probability (P = 0.95); Compare the results of contents of test samples of 2 analysts or 2 different testing days of 1 analyst by “T test two sample assuming unequal variances” with the probability 95%.
- If the test results comply with the specification, this material will be accepted to be used as working standard. Measurement results are recorded and establish COA, analysis report …
A full record of working standard includes:
1. COA of working standard.
2. Analytical record of working standard (including spectra, chromatograms, weighing data, etc.)
3. COA of the primary standard
4. COA of raw materials from the manufacturer
5. COA of raw materials by QC Dept.
3.4 Labeling – storage
Content in label normally includes:
- Name of working standards
- Bottle number
- Batch number
- Assay
- LOD / water content
- Date of preparation
- Use before
- Storage conditions
Storage regulation:
- Store the working standards in light-resistant glass vials, closed with rubber bungs and sealed with the Aluminium / Paraffin seals.
- The quantity for each vial depends on each quantitative method, from several tens to several hundreds of milligrams.
- Store all additional vials of working standards in A.C. controlled area (below 25°C) or in a refrigerator (between 2 to 8°C) or at ≤ 00C, following the raw materials storage regulation.
- The whole packaging should be carried out in the defined environmental condition
3.5 Stability test to determine shelf life
- The shelf life of working standards is determined from validation data of 3 batches of working standards from 1 a stable, quality supply of raw material. However, after determining the shelf-life of 1 batch of working standard, temporarily accept this shelf-life and add gradually until getting enough 3 batches of working standard as the above requirement. Thus, after there are validation results of the first 3 batches, the shelf-life of working standard has been established. From the 4th batch of working standards onwards, no further validation of the shelf life is required, unless there .
- Monitor the stability of the working standard used to check product quality with the frequency of determining the shelf life of the first batch of working standards: initial sample, 1-month sample, 3-month sample, 6-month sample, 9 month sample and 12 month sample. Frequency of determining the expiry date of the second batch of working standards; 3rd: original sample, 6 month sample, 12 month sample.

4. Note for the analyst
- The analyst should ensure that the working standard has not expired.
- The analyst should keep the standards out of storage for as short a time as possible to protect them from moisture and light.
- Equilibrate the working standard to room temperature at least 30 minutes after being stored in refrigerators.
- Use required quantities and do not put back the working standard in a vial.
- After usage of Working standard, reseal the vial properly and kept at the allocated area.
- Dispose of the working standard after its expiry as per the SOP.



