
T-Shape & Business Acumen – Core Competences And Values Of HR Professionals
6 October, 2022
Measures For Drug Price Management In Vietnam
26 October, 2022The current view of the world’s regulatory authorities is that Process Validation (PV) demonstrates the adequacy and robustness of the manufacturing control strategy and gives continuing assurance that the process is under control. It is defined in the November 2016 EMA Guideline on process validation as:
“Process validation should not be viewed as a one-off event. Process validation incorporates a lifecycle approach linking product and process development, validation of the commercial manufacturing process, and maintenance of the process in a state of control during routine commercial production.”
It is also defined in the 2011 FDA PV Guidance as:
“…the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering a quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process.”
In comparison to the traditional or historic approach, this new (enhanced) approach to PV consists of three stages, as shown in Figure 1.1. To provide validation throughout the product lifecycle, the science and risk-based approach should be applied and integrates product development knowledge with a process performance and product quality monitoring system.
Figure 1.1: Process Validation Overview – Process Validation Lifecycle – the Enhanced Apache
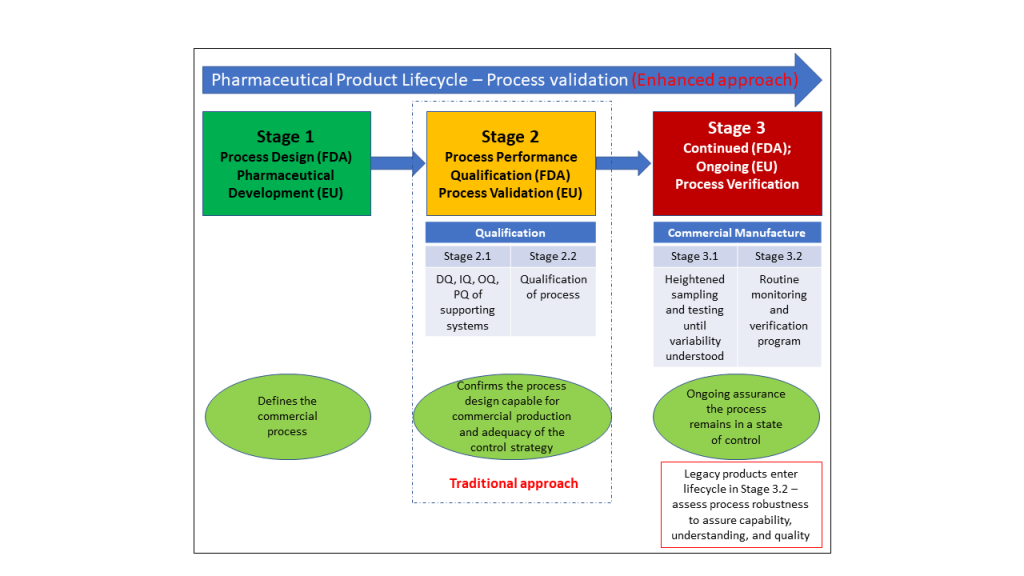
Each phase of the process validation lifecycle is unique from the typical course of product development activities. Stage 1, often known as the pharmaceutical development stage, is where the commercial process is defined. Stage 2 (process validation) involves evaluating and verifying the process design to see if the manufacturing process is capable and reproducible. Stage 3, also known as ongoing process verification, is where continued assurance of a state of control is obtained through routine commercial batch monitoring.
An effective process validation program ensures quality, safety, and efficacy are designed and built into the product through its lifecycle. This is achieved by ensuring all manufacturing steps are controlled, measured, and quantified by finished product quality attribute specifications. To effectively use data from all stages, sound scientific principles, strong knowledge management, documentation, and pharmaceutical quality procedures are essential. This represents a significant change from previous guidance and requirements for declaring a process validated. Sources of variation, the impact of the variation on the process and quality attributes, and control of the variation are required.
PV is no longer considered a one-time event a lifecycle approach is expected by most global markets. PV success relies on establishing a comprehensive science-based process design that focuses on understanding sources of variability to achieve product realization.
References
1. US FDA. Guidance for industry, process validation (2011): general principles and practices.
2. European Commission (EC). Guidelines on good manufacturing practice for medicinal products, Annex 15. 2015.
3. ISPE Good Practice Guide: Practical Implementation of the Lifecycle Approach to Process Validation.



